For patients: questions to ask (and how HealthScout helps)
August 31, 2025 John Emmett Worth
Emmett is a clinical research coordinator at Stanford who helps build and test HealthScout.
During my onboarding as a clinical research coordinator, I was struck by the breadth of coordination that goes into even a single blood collection. Each task—from processing a kit to logging data—represents the combined efforts of many different hands, each pushing the study forward.
Helping build HealthScout expanded this perspective. The coordination I witnessed in just a handful of trials scales dramatically when you consider hundreds of potential trials, each with its own logistics, collaborators, and layers of complexity. Through nearly a year of enrolling patients in clinical trials, I’ve seen firsthand how patients struggle to navigate this system. That experience inspired me to distill the five most important questions every patient should ask when considering a clinical trial. These same questions have shaped HealthScout’s design—always returning to the core idea of making trial access more transparent and navigable.
Below are five questions to ask your clinical research team—and how HealthScout can help you answer them.
1. What’s the purpose?
- Why it matters: Some trials aim to shrink tumors. Others explore safety. Not every “match” is the right match for your specific goals. It’s important to get a thorough understanding from the trial’s research team about both the background (why the trial was created) and the foreground (what it hopes to prove).
- How HealthScout helps: We surface this information up front. The trial’s “Goal” appears prominently for every match, and we summarize what the study is trying to do—no detective work required. The “Additional resources” section in each listing links to related news or recent research updates for helpful context.
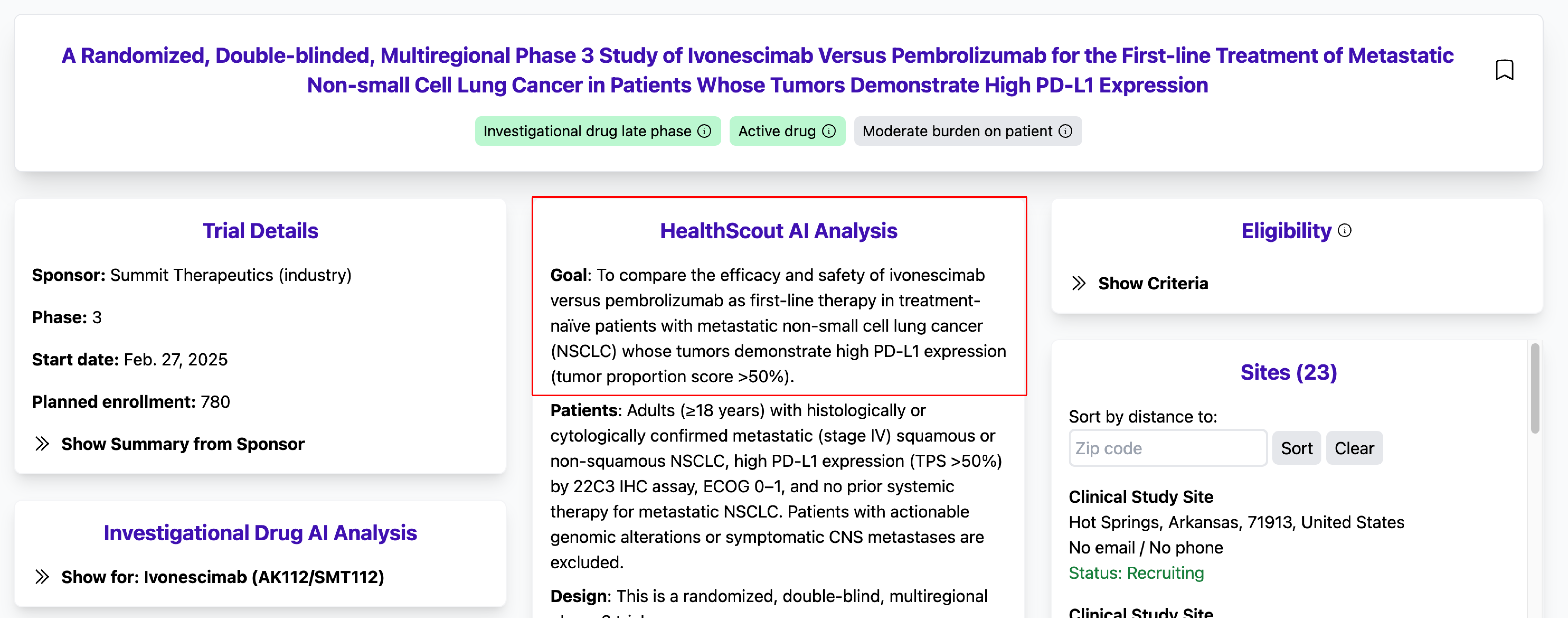
2. What makes me eligible—why me?
- Why it matters: Trial criteria can feel like they’re written in code. If a study wants you, you should know exactly why—and feel confident that you’re a fit.
- How HealthScout helps: The AI Picks tab explains, in plain language, why you might be a good fit. The trial’s Eligibility section also breaks down criteria into those we used for matching and those you should review carefully because they depend on information we don’t have (for example, other medical issues or lab values).

3. What are my responsibilities as a participant?
- Why it matters: A trial might be medically promising but logistically unworkable. Weekly biopsies, lengthy infusions, or out‑of‑state travel can take a toll—especially when you’re also navigating treatment and everyday life.
- How HealthScout helps: We flag potentially burdensome aspects—such as frequent visits, invasive procedures, and travel distance—so you can balance opportunity with practicality.
4. What stage is the study—phase 1, 2, or 3?
- Why it matters: The phase of a trial tells you a lot about what to expect. Early‑phase studies may focus on safety or dosage, while later phases test how well the treatment works compared to existing options. Understanding the phase can help you decide whether a trial aligns with your goals and comfort level.
- How HealthScout helps: Every trial listing clearly labels the study phase. It might seem like a small detail, but when comparing options it can meaningfully influence your decision.
5. What are my options?
- Why it matters: This question sits at the heart of HealthScout’s vision. Even if one trial looks promising, is it the best fit? Faced with hundreds of options, how do you make an informed decision?
- How HealthScout helps: AI Picks sorts through the noise, filtering trials based on your medical details and preferences. The result is a clear, prioritized list with useful context—making complex data simpler and more actionable. Treat it as a starting point for your own research and conversations with your doctor.
Conclusion
As someone who’s worked behind the scenes of clinical research, I believe patients deserve transparency, clarity, and options. The right trial can change everything—and asking the right questions is the first step.
